Climate emergency and humanity's challenges
In the context of a climate emergency, humanity faces two major challenges: obtaining potable water and storing renewable energy. Traditionally, these challenges have been addressed separately through reverse osmosis desalination for producing potable water and the use of batteries for energy storage. However, combining these solutions could represent a significant advance in the energy transition. An emerging technology that promises to integrate these solutions is redox flow desalination (RFD), a field where New York University has recently made considerable advances.

What are redox flow batteries?
To understand redox flow desalination, it is important to first know the concept of redox batteries. These batteries store energy in liquid solutions called electrolytes, which contain chemical compounds capable of changing from an oxidized to a reduced state and vice versa. During operation, two types of electrolytes are pumped from separate tanks through a central electrochemical cell. In this cell, the electrolytes interact through an ion exchange membrane, generating electricity that can be used or stored. Redox batteries are especially useful for renewable energy storage due to their ability to withstand many charge and discharge cycles without significant degradation.
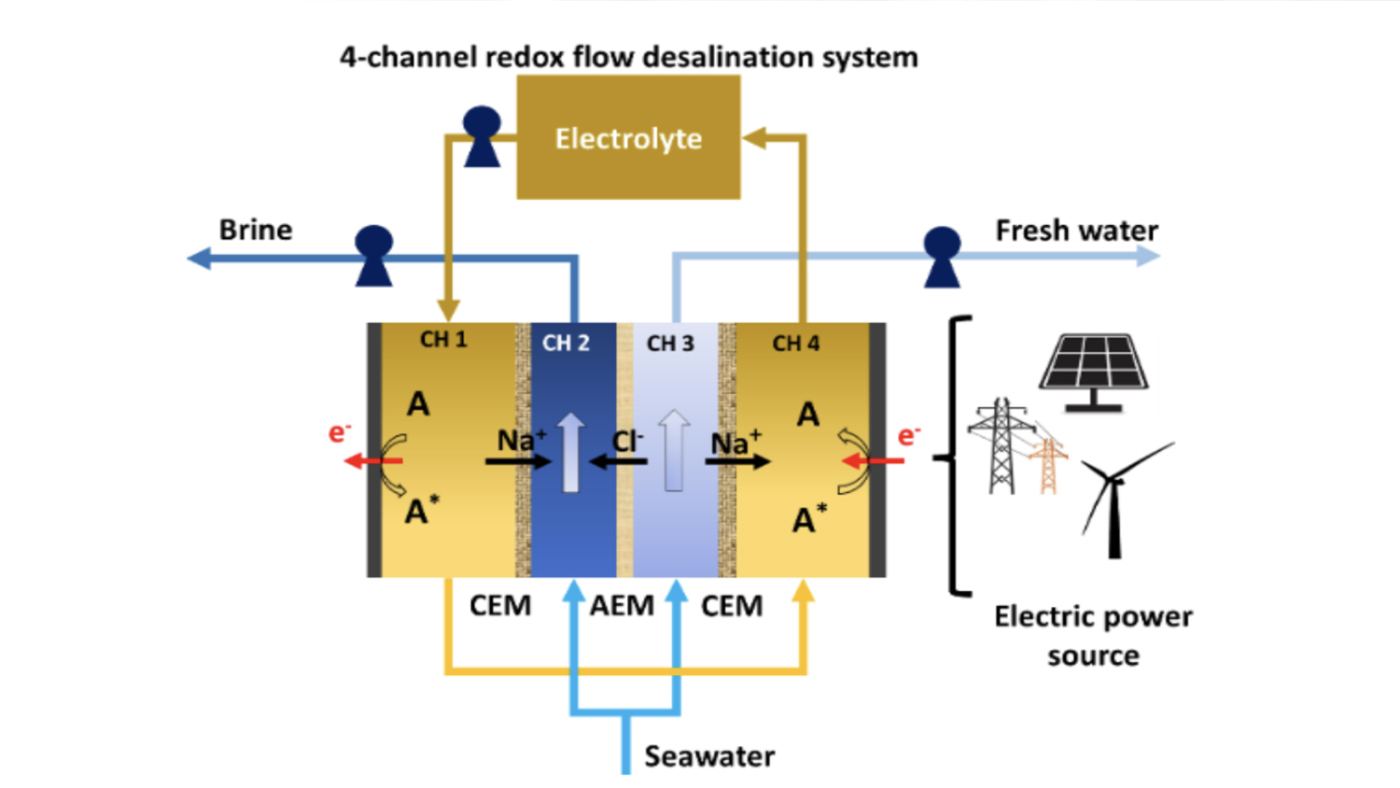
Schematic for a four-channel RFD in single-pass mode with an A/A*, depicting electrochemical reactions of dissolved redox species in conducting salt solutions and channels separated by a cation exchange membrane (CEM) and an anion exchange membrane (AEM).
The new generation of desalinating redox batteries
Redox flow desalination (RFD) is an innovative technique that combines water desalination and energy storage in one system. Instead of using traditional methods, RFD circulates saline solutions and redox agents through electrochemical cells. Ion exchange membranes allow for selective ion transfer, extracting salt from seawater and producing fresh water. In addition to producing potable water, the RFD process allows excess renewable energy to be stored in the redox molecules, which can then be released when needed, functioning like a battery.
Advances at New York University
Researchers at New York University's Tandon School of Engineering have achieved a significant breakthrough in redox flow desalination (RFD), an emerging electrochemical technique that not only converts seawater into potable water but also efficiently stores renewable energy. In a study published in Cell Reports Physical Science, the NYU Tandon team, led by Dr. André Taylor, professor of chemical and biomolecular engineering and director of DC-MUSE (Decarbonizing Chemical Manufacturing Using Sustainable Electrification), has increased the efficiency of the RFD system. They optimized fluid flow rates, increasing the salt removal rate by approximately 20% while simultaneously reducing energy consumption.
The RFD system developed by NYU uses a structure of four channels separated by ion exchange membranes (IEM). Increasing the flow rate in the electrolyte channels reduces resistance at the electrolyte-membrane interface, significantly improving salt removal and energy efficiency. For example, increasing the flow rate from 5 to 50 mL/min has allowed the salt removal rate to increase 16.7 times and reduce energy consumption.
Schematic of Professor Taylor's 4-channel redox flow desalination system, interpreted by the Dall-E AI.
Features of the RFD system
The redox flow desalination (RFD) system offers multiple benefits:
To understand redox flow desalination, it is important to first know the concept of redox batteries. These batteries store energy in liquid solutions called electrolytes, which contain chemical compounds capable of changing from an oxidized to a reduced state and vice versa. During operation, two types of electrolytes are pumped from separate tanks through a central electrochemical cell. In this cell, the electrolytes interact through an ion exchange membrane, generating electricity that can be used or stored. Redox batteries are especially useful for renewable energy storage due to their ability to withstand many charge and discharge cycles without significant degradation.

Schematic for a four-channel RFD in single-pass mode with an A/A*, depicting electrochemical reactions of dissolved redox species in conducting salt solutions and channels separated by a cation exchange membrane (CEM) and an anion exchange membrane (AEM).
The new generation of desalinating redox batteries
Redox flow desalination (RFD) is an innovative technique that combines water desalination and energy storage in one system. Instead of using traditional methods, RFD circulates saline solutions and redox agents through electrochemical cells. Ion exchange membranes allow for selective ion transfer, extracting salt from seawater and producing fresh water. In addition to producing potable water, the RFD process allows excess renewable energy to be stored in the redox molecules, which can then be released when needed, functioning like a battery.
Advances at New York University
Researchers at New York University's Tandon School of Engineering have achieved a significant breakthrough in redox flow desalination (RFD), an emerging electrochemical technique that not only converts seawater into potable water but also efficiently stores renewable energy. In a study published in Cell Reports Physical Science, the NYU Tandon team, led by Dr. André Taylor, professor of chemical and biomolecular engineering and director of DC-MUSE (Decarbonizing Chemical Manufacturing Using Sustainable Electrification), has increased the efficiency of the RFD system. They optimized fluid flow rates, increasing the salt removal rate by approximately 20% while simultaneously reducing energy consumption.
The RFD system developed by NYU uses a structure of four channels separated by ion exchange membranes (IEM). Increasing the flow rate in the electrolyte channels reduces resistance at the electrolyte-membrane interface, significantly improving salt removal and energy efficiency. For example, increasing the flow rate from 5 to 50 mL/min has allowed the salt removal rate to increase 16.7 times and reduce energy consumption.

Schematic of Professor Taylor's 4-channel redox flow desalination system, interpreted by the Dall-E AI.
Features of the RFD system
The redox flow desalination (RFD) system offers multiple benefits:
- Scalability and Flexibility: The system provides a scalable approach to energy storage, allowing for efficient utilization of intermittent renewable sources such as solar and wind energy.
- Reduction in Dependence on Conventional Grids: RFD can reduce dependence on conventional power grids and promote the transition to a carbon-neutral desalination process.
- Improvement in System Efficiency: The integration of redox flow batteries with desalination technologies improves system efficiency and reliability.
System operation
The RFD process involves splitting incoming seawater into two streams: the salinization stream and the desalination stream. Additionally, two channels house the electrolyte and the redox molecule, separated by a cation exchange membrane (CEM) or an anion exchange membrane (AEM). In a reverse operation, where the brine and fresh water mix, the stored chemical energy is converted into renewable electricity.
Applications and potential
The RFD system can serve as a unique form of "battery," capturing excess energy from solar and wind sources to release it on demand. This duality demonstrates its potential not only in desalination but also as an innovative contribution to renewable energy solutions.
Collaboration and recognitions
The success of this project is largely attributed to Stephen Akwei Maclean, the first author of the paper and a Ph.D. candidate in chemical and biomolecular engineering at NYU Tandon. Maclean designed the system architecture using advanced 3D printing technology at the NYU Maker Space.
The NYU Tandon research team includes Syed Raza, Hang Wang, Chiamaka Igbomezie, Jamin Liu, Nathan Makowski, Yuanyuan Ma, Yaxin Shen, and Jason A. Röhrl. Additionally, Guo-Ming Weng from Shanghai Jiao Tong University in China collaborated as a crucial team member.
Future implications
Although more research is needed, the findings of the NYU Tandon team suggest a promising pathway towards a more cost-effective RFD process. This breakthrough is crucial in the global quest for potable water, especially in the context of climate change and population growth.
This work aligns with the mission of DC-MUSE, a collaborative initiative established at NYU Tandon to advance research that reduces the environmental impact of chemical processes through the use of renewable energy.
Publications and achievements
This achievement marks the 100th publication of the Taylor Transformative Materials and Devices Laboratory, originally established at Yale in 2008 and relocated to NYU Tandon in 2018. The lab focuses on developing innovative materials and devices for energy conversion and storage, reflecting Taylor's enduring commitment to transformative research in the field.
For more information about this research and other innovative projects at NYU Tandon, visit NYU Tandon School of Engineering.
Did you like this post? Check out our latest current news!



